Introduction
Cell viability measurement is an essential aspect of routine cell culture processes. It involves assessing the number of viable cells in a given population, which is critical for various applications, including cell-based assays, gene expression studies, and protein analysis.
In this guide, we’ll explore the different methods of cell viability measurement and their applications. We’ll also discuss the importance of this technique in different fields and how it can affect your research.
In routine cell culture, cell viability measurement is used to monitor the health and growth of cells. By regularly measuring the viability of cells, researchers can ensure that their cells are healthy and growing optimally. This is particularly important in long-term experiments or when working with sensitive cells that require specific growth conditions.
Cell viability measurement is also useful in assessing the quality of the cells before starting experiments. Poorly cultured cells can lead to inaccurate results, so it is essential to ensure that the cells are viable and healthy before conducting experiments. By measuring cell viability, researchers can identify any issues with the cell culture process and make adjustments to improve cell growth and health.
In addition, cell viability measurement can be used to optimize experimental conditions. By measuring cell viability under different experimental conditions, researchers can determine the optimal conditions for cell growth and maximize the efficiency of their experiments.
In drug discovery, cell viability measurement is used to screen potential drug candidates and determine their effect on cell viability. By measuring the viability of cells exposed to different drug concentrations, researchers can identify compounds that have the desired effect on the target cells while minimizing the impact on healthy cells.
Cell viability measurement is also used to determine the optimal dosage of a drug candidate. By measuring the viability of cells exposed to different concentrations of a drug, researchers can determine the optimal dosage that maximizes the therapeutic effect while minimizing potential toxicity.
In addition, cell viability measurement is useful in identifying potential side effects of a drug candidate. By measuring the viability of cells exposed to different concentrations of a drug, researchers can identify potential toxicity and side effects and make adjustments to the drug formulation or dosage to minimize these effects.
Overall, cell viability measurement is a critical technique in drug discovery and development. By accurately measuring the viability of cells, researchers can identify potential drug candidates, optimize their efficacy, and minimize potential side effects, leading to the development of safe and effective drugs.
In toxicology studies, cell viability measurement is used to assess the impact of a substance on cell viability. By measuring the viability of cells exposed to different concentrations of a substance, researchers can determine the potential effects of the substance on living organisms.
Cell viability measurement is also useful in identifying the mechanism of toxicity of a substance. By measuring the viability of cells exposed to different concentrations of a substance and analyzing the changes in cell morphology and function, researchers can identify the mechanism of toxicity and better understand how the substance impacts living organisms.
In addition, cell viability measurement is useful in evaluating the efficacy of potential treatments for toxic substances. By measuring the viability of cells exposed to different concentrations of a potential treatment, researchers can determine the optimal dosage and treatment strategy to minimize the impact of the toxic substance.
Overall, cell viability measurement is a crucial technique in toxicology studies. By accurately measuring the viability of cells, researchers can identify the potential toxicity of substances, evaluate their effects on living organisms, and develop effective treatment strategies to minimize their impact.
Methods of Cell Viability Measurement
There are several methods for cell viability measurement, including:
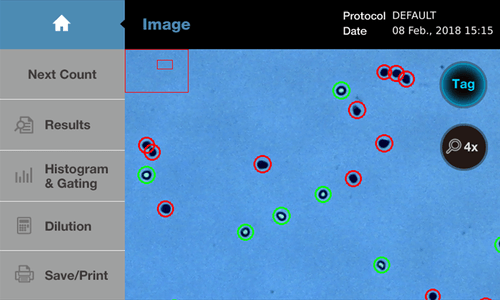
Trypan blue staining is the most commonly used method for measuring cell viability. This method is based on the principle that healthy living cells have a cell membrane that prevents the penetration of dyes like trypan blue. In contrast, cells with dead or damaged membranes allow the dye to penetrate, binding to intracellular proteins and staining the cells.
Trypan blue dye is a water-soluble molecule with a negative charge and a size of approximately 960 Da. Due to its size and charge, trypan blue cannot easily penetrate the lipid bilayer of the cell membrane. Molecules that can penetrate the cell membrane of living cells are generally smaller than 600 Da, and molecules larger than this size have difficulty entering cells due to their selective permeability.
Trypan blue staining is typically carried out by mixing 0.4% Trypan blue staining with a cell suspension in a 1:1 ratio. The staining process is rapid, so incubation periods longer than a few minutes are not recommended. The stained cell suspension can then be counted under a microscope using a hemocytometer. Hemocytometers have a fixed volume, making it possible to calculate the concentration of cells in the sample and measure viability.
Nowadays, most laboratories use automated cell counters to perform these steps automatically. Automated cell counters use image analysis to accurately distinguish between live and stained dead cells, reducing user error and fatigue. (For more information on the reasons to invest in automated cell counters, see “Top 10 Reasons to Invest in Automated Cell Counters for Accurate and Efficient Research”
Although trypan blue staining is widely used to evaluate cell viability, it has several drawbacks.
Firstly, the accuracy of the results can be affected by the state of the cells. For example, cells with temporarily permeable cell membranes can be stained even though they are not dead.
Secondly, trypan blue staining without an automatic cell counter requires manual counting of cells and calculation of the percentage of surviving cells, which can be slow and inefficient, especially for large sample sizes.
Thirdly, trypan blue dye itself can be toxic to cells, potentially affecting the sample if exposed for long periods.
Fourthly, when counting primary cells collected from living tissue, it can be difficult to distinguish red blood cells from stained dead cells. This can lead researchers to mistakenly count red blood cells as dead cells and underestimate the survival rate of the sample.
Finally, the toxicity of trypan blue not only affects the results of cell counting but also poses health risks to users who handle the dye.
Many European countries have already begun to restrict the use of trypan blue in laboratories due to its toxicity. Erythrosin B is an alternative dye with significantly lower toxicity that is already used to measure cell viability and count cells. Erythrosin B has a molecular weight of 879.9 Da, which prevents it from penetrating the cell membrane of living cells and only staining dead cells, similar to the principle of trypan blue staining.
Fluorescence dye staining is a method of distinguishing between living and dead cells using fluorescent dyes, which are combined in various ways with dyes that have permeability to the cell membrane and dyes that do not. For example, the widely used AOPI staining method combines acridine orange (AO), which has permeability to living cells, and propidium iodide (PI), which cannot penetrate the cell membrane, to stain cells. AO stains both living and dead cells, while PI stains only dead cells, allowing the calculation of cell survival rates. Alternatively, there is a method of counting only living cells by using dyes that selectively emit light only in living cells among dyes with good permeability to cells. We will describe various fluorescence dye staining methods for measuring cell survival rates below.
1. AOPI staining (Acridine orange/Propidium Iodide Staining)
Fluorescence dye staining, such as acridine orange/propidium iodide (AOPI) staining , is another method commonly used for measuring cell survival rates. This method uses two fluorescent dyes, acridine orange and propidium iodide, which can distinguish between living and non-living cells. When using AOPI staining to measure cell survival rates, living cells emit green fluorescence, while dying or damaged cells emit red fluorescence. Using a fluorescence microscope or flow cytometry, viable cells and non-viable cells can be distinguished, and the proportion of viable cells in a cell population can be calculated.
Acridine orange (AO) is a small molecule with a molecular weight of 265 Da that can pass through the cell membrane relatively easily and selectively binds to nucleic acids within cells. When AO is bound to DNA, it has a peak excitation at approximately 500 nm and a peak emission at 525 nm.
On the other hand, propidium iodide (PI) has a molecular weight of 668 Da and cannot penetrate the cell membrane as easily as AO. PI also binds to nucleic acids within cells and has peak excitation at 535 nm and peak emission at 617 nm when bound to DNA.
When both AO and PI are used to stain cells, AO can penetrate all cells and bind to nucleic acids, while PI only binds to nucleic acids in dead cells due to its inability to penetrate live cell membranes. AO fluorescence can be observed after excitation at around 500 nm and emission at around 530 nm, while fluorescence from PI is observed after excitation at 530 nm and emission at around 620 nm. Since live cells allow AO penetration only, the fluorescence of PI is not observed in live cells. In dead cells, both dyes can penetrate and theoretically both AO and PI fluorescence can be observed. However, due to the FRET phenomenon, where AO emission is absorbed to PI, only the fluorescence of PI is selectively observed in dead cells. This means that the fluorescence of AO is not observed in dead cells when AO and PI are used together. Using a fluorescence microscope or an automatic cell counter that adopts the principle of a fluorescence microscope, green fluorescence of AO is observed in living cells, while red fluorescence of PI is observed in dead cells.
2. FDA/PI staining method
FDA (Fluorescein diacetate) is a derivative of fluorescein, a representative fluorescent dye that emits green fluorescence, but it is designed to not emit fluorescence under normal conditions. FDA is a low molecular weight compound with a molecular weight of 416 Da that can relatively freely penetrate the cell membrane, and is decomposed by intracellular esterase enzymes to release fluorescein. Therefore, if living cells are stained with FDA, the FDA that has penetrated the cell membrane will be decomposed by intracellular esterase and emit green fluorescence. In contrast, dead cells do not have esterase activity and FDA remains intact without emitting fluorescence.
FDA is used in conjunction with PI, which can selectively label dead cells, to easily measure cell survival by observing green fluorescence from living cells and red fluorescence from dead cells.
Another dye that operates similarly to FDA is Calcein-AM, which is also decomposed by intracellular esterase to emit fluorescence.
3. MTT Assay
MTT assay is a method for measuring cell viability based on the reduction of the yellow tetrazolium salt MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to purple formazan crystals by mitochondrial dehydrogenases in living cells.
The typical procedure for performing MTT assay involves seeding cells in a well plate, allowing them to attach and grow, treating them with the desired drug, adding MTT solution to each well, and incubating for several hours to allow MTT to be metabolized. The generated formazan crystals produced by living cells are then measured quantitatively. To dissolve the formazan for quantitative analysis, solutions such as DMSO, acidic ethanol, or acidic SDS are commonly used. The dissolved formazan can be measured for its absorbance at 570nm using a microplate reader or spectrophotometer, and the amount produced is proportional to the number of surviving cells.
The biggest advantage of MTT assay is that cell viability can be measured relatively quickly and easily while cells are attached. However, this method may not accurately reflect the viability of cells with damaged or dysfunctional mitochondria, as it is based on mitochondrial function. In addition, this method can be influenced by factors such as cell density, incubation time, pH, and can affect the accuracy of the results. Above all, the biggest drawback of MTT assay is that a control group is required. While cell viability in the treatment group can be easily evaluated compared to the control group, cell viability cannot be calculated without a control group. For example, the cell viability of cells currently in culture cannot be measured with MTT assay.
The ATP assay is a rapid method for cell viability measurement. It involves the measurement of ATP levels in viable cells using a luciferase-based assay. The luminescence produced by the reaction is directly proportional to the amount of ATP present in the cells, allowing for the measurement of cell viability.
Cell viability measurement is a critical technique in various fields, including routine cell culture process, drug discovery, and toxicology. By measuring the viability of cells, researchers can monitor cell health of cultured cells, identify potential drug candidates, and assess their efficacy and potential side effects.
References
Kim et al., Application of a non-hazardous vital dye for cell counting with automated cell counters, Anal Biochem, 2015
A W Krause, et al., Fluorescent erythrosin B is preferable to trypan blue as a vital exclusion dye for mammalian cells in monolayer culture, J Histochem Cytochem, 1984